Which is stronger than vinegar or vinegar essence. Characterization and application of acetic acid
Vinegar is a product of microbiological synthesis. It is obtained from food-grade alcohol-containing material thanks to the acetic acid bacteria. This substance has been known since ancient times and has found wide application in cooking, household and industry. There are many varieties of this product, such as white vinegar. What is it and what is it used for?
History reference
The age of production and use of vinegar is quite comparable to that of winemaking. The first mention of this substance, which was found, dates back to 5000 BC. e. It was made in ancient Babylon from dates, from which they also made wine.
Its scope was wide enough. Vinegar served as a condiment, antiseptic, and disinfectant.
Assortment range
By origin, all vinegar is divided into two types: natural and synthetic. Both can be used in the food industry. Synthetic is found not only in its pure form, but also with various flavors. In this role, herbal extracts are used, which are also often used as condiments. Natural alcoholic vinegar, which is obtained from ethyl alcohol, can also be flavored.
Natural vinegar can be apple cider, wine, balsamic, and malt.
Table vinegar and white vinegar. What is the difference?
Many housewives believe that table vinegar, which can be found in any kitchen, is white vinegar. That is, these are synonymous names for the same substance. In fact, this point of view is erroneous.
What makes white vinegar different from table vinegar is that table vinegar is a diluted solution of vinegar essence that you can buy at the store. Then, depending on the required concentration, it is diluted with less or more water. White, on the other hand, is a product of beer wort fermentation.

How vinegar is made
To obtain vinegar, natural raw materials are needed. In this capacity, a variety of fruit juices, grape juice and remnants of wine material can act. And also rectified ethyl alcohol and products of its processing. Alcohol is fermented by acetic acid bacteria. The complete oxidation process is not allowed, otherwise complete decomposition into water and carbon dioxide will occur.
After the oxidation process is completed, it is required to clean, pasteurize and pour the finished product. In some cases, the substance must be diluted.
White vinegar
If you look at what white vinegar looks like, it can be confused with regular table vinegar. Malt vinegar has a yellowish tint, but after being purified it becomes colorless. Since it has a rather strong odor and a sour taste, it is not used for dressing salads or for consumption in finished form... The most common areas of its use are marinade making and preservation.
But beer wort is not the only material from which this type of vinegar is made. Then white vinegar - what is it when it is obtained from other raw materials? Most popular among culinary specialists vinegar... Its white variety is the result of fermentation of the by-products of wine production.

The base is white wine. Thanks to the softer and good taste it is often used in a wide variety of fish and meat dishes. It is used for refueling vegetable salads making various sauces.
What is balsamic vinegar
It takes a long time to make balsamic vinegar, a large number of a certain grape variety and adherence to the entire manufacturing technology, which is more complicated than it might seem. But due to its taste characteristics, it is considered the most valuable type of vinegar.
It is produced in one region of Italy - in Modena. From berries white grapes juice is squeezed out, which is then subjected to the caramelization process. Since sugar darkens when heated, the substance acquires a dark color and a special taste. Unlike all other vinegars, balsamic vinegars are aged in wooden barrels for at least 12 years.
Since the complexity of manufacturing seriously affects the price, more simple options vinegar, for the manufacture of which the technology is not so strictly adhered to. Moreover, this is a completely high-quality product that can be used for dressing salads, adding to sauces, sprinkling with meat and desserts.
Balsamic Vinegar Variations
Over the years, several new interpretations of the classic balsamic vinegar have emerged, through a variety of flavors. To do this, vinegar is infused with lemon, raspberry or cherry.
There is also white balsamic vinegar, which is produced using a different technology.

To prevent the color from becoming dark, the grapes do not caramelize, and the vinegar itself is aged in steel barrels, not wood. The result is a product with a very beautiful rich golden hue. It is used when the light color of the vinegar will look much more advantageous in the dish than the dark one.
Uses of vinegar
White vinegar is not as commonly used in cooking as wine or apple cider vinegar. Due to its specific taste, it is suitable for preserving fruits and vegetables.

By the way, sometimes according to culinary recipes it is necessary to add white wine, but when it is not there, what can be used instead? White vinegar will come to the rescue, you just need to add a little sugar to it, and the result will not disappoint.
It is well suited for solving many everyday problems. For example, it will perfectly clean plastic or glass dishes, the surface of the stove. Mixing baking soda with vinegar helps keep your plumbing clean without spending a lot of time and energy on it.
This substance is also great for dealing with strong and unpleasant odors. To do this, you need to moisten a rag with it and wipe the surfaces in those places where you need to get rid of them. White vinegar is also suitable for cleaning cutting boards, the use of which will not only get rid of odors, including fishy ones, but also disinfect the surface.

To make the kettle shine, you need to add a little product to the water and boil it. To clean the iron, pour the vinegar inside. Leave it with the included steaming mode in an upright position. After 10 minutes, the remaining liquid should be poured out and the compartment where the water is poured should be rinsed well.
White vinegar is very effective for solving a large number of problems with things. What is it if not a budget bleach and stain remover? To easily get rid of underarm sweat marks, you will need to moisten the stains with liquid and can be washed after 10 minutes. If there are many stains on things that cannot be washed, then you will need to make a solution of vinegar and water (1: 1) and soak the clothes in it for several hours. Vinegar perfectly removes unpleasant odors from things, returns them to their original brightness, and acts as an antistatic agent. Therefore, it should be added to the washing machine every time before starting the wash.
White vinegar - what is it? This is a wonderful product of natural origin, which can be used not only in the kitchen, but also in everyday life.
It is one of the basic products obtained in the process of industrial organic synthesis. Acetic acid has no color, but it has a specific smell and taste; it is obtained through the oxidation of a certain aldehyde; due to its chemical properties, it is capable of causing significant harm to humans, therefore the liquid is used only in the form of aqueous solutions. More than half of the product produced is spent on the manufacture of polymers, as well as derivatives of vinyl acetate and cellulose.
What is acetic acid
It is a synthetic product formed by the fermentation of ethanol and carbohydrates or after the natural souring of dry wine varieties. Ethanic acid takes part in metabolic processes in the human body. The acidic liquid, in addition, is used for the preparation of preservation, marinades. Certain properties of the product make it indispensable in a variety of chemical compounds, household products.
Formula
Acetic acid contains vinegar 3-9% vinegar and 70-80% vinegar essence... The salts and esters of the product are called acetates. Common vinegar used in cooking contains malic, ascorbic, acetic, and lactic acids. About 5 million tons of ethanic acid are produced annually in the world. Its chemical formula is as follows: C2H4O2.
Receiving
What is acetic acid made of today? To obtain a liquid for technical purposes, black wood powder is used, which contains a large amount of resinous substances. The most advantageous chemical method for obtaining the product is the oxidation of ethanal or acetaldehyde, which is obtained in industry either by hydration of acetylene with mercury salts (the method is called the Chugaev reaction) or by oxidation of ethyl alcohol over hot copper. Acetaldehyde is independently oxidized by oxygen and transformed into acetic acid.
The acetic acid solution is transported over different distances in road or rail tank cars made from special types of stainless steel materials. In warehouses, liquid is stored in sealed containers, containers, barrels under sheds or in special rooms. It is allowed to fill in and store acid in a polymer container for a maximum of one month.
Concentration
Acetic acid solutions that are used by the food industry, household cooking, conservation are called vinegar and vinegar essence. Absolute concentrated acid is called glacial, because when it freezes, it transforms into a mass that resembles ice in structure. Different concentrations of acetic acid determine the following product classification:
- essence (contains 30-80% acid, is a component of medicines for itching, fungi);
- ice (solution 96%, used to remove corns, warts);
- table vinegar (has a concentration of 3, 6 or 9%, is actively used for domestic purposes);
- acetate substance (acid ester);
- natural Apple vinegar(has a low acid percentage, used by cosmetologists, chefs);
- balsamic vinegar (a table product infused with certain herbs).
Properties
The transparent liquid has a pungent odor and a density of 1.05 g / cm2. Physical properties acetic acid cause it to freeze at a temperature of 16.6 degrees, while the substance takes on the form of transparent crystals resembling ice (because of this, a concentrated acidic liquid is called ice cold). Acid has the ability to actively absorb moisture from the air, it can neutralize basic oxides and hydrates, and, in addition, displace carbon dioxide from carbon dioxide.
The effect of acetic acid on the human body
The vinegar product is classified as a substance with the third hazard class due to its flammability and hazardous effects on the body. For any work with the substance, specialists use modern protective equipment (gas masks). Even the E-260 food additive can be toxic to the body, but the degree of its effect depends on the concentration and quality of the product. The dangerous effect of vinegar on the body is possible when the acidity is above 30%. If the concentrated substance interacts with the skin / mucous membranes, severe chemical burns will appear on the body.
With judicious use of the product, vinegar will help eliminate many diseases and cosmetic defects. So, the vinegar product is used for the treatment of colds and rheumatism as a preparation for grinding. The acidic liquid, in addition, has a bactericidal effect: a natural antiseptic helps to destroy fungi and other pathogenic flora with angina, pharyngitis, thrush. Vinegar is good for hair because it is an excellent anti-dandruff remedy. For the skin, the liquid is used in cosmetic wraps and as a remedy for itching after an insect bite.

Overdose
The effect of a vinegar product on the human body resembles the effect of nitric, sulfuric or hydrochloric acids, with the main difference being the surface effect of vinegar. The lethal dose of the product for humans is 12 ml: this amount is equal to about a glass of vinegar or 20-40 ml of essence. Acetic vapor, when it enters the lungs, causes the development of pneumonia with complications. Other symptoms of an overdose are:
- hemorrhage of the liver;
- tissue necrosis;
- burns of internal organs;
- ulceration of the digestive tract;
- nephrosis with concomitant death of renal cells.
The use of acetic acid
The acidic liquid is widely used in various fields. It is indispensable for pharmacology, as it serves as a component of aspirin, phenacetin and other drugs. During nitration, aromatic amines NH2 groups are protected by introducing an acetyl group CH3CO - this is also a common reaction in which vinegar is involved. The product plays an important role in the production of acetone, cellulose acetate, various synthetic dyes.
The production of various perfumes and non-combustible types of films is not complete without a product. An acidic liquid is often used in the food industry, as an additive for E-260. At the same time, household cooking and canning cannot do without vinegar. When dyeing, the main types of acid salts perform the function of special mordants, providing a strong bond of textile fibers with the dye. Acetic salts are often used to eradicate the most persistent pests.
In medicine
In pharmacology and the medical field, liquid is used as the basis of medicines, for example, acetylsalicylic acid (aspirin). In addition, acetic acid salts of lead and aluminum are obtained from it, which act as astringents and are used to treat inflammatory processes of various etymologies. Vinegar has antipyretic, anti-inflammatory, analgesic effects, so it is used for headaches, fever, neuralgia, etc.
The acidic substance is often combined with other drugs in folk medicine for the treatment of many pathologies - polyarthritis, lichen, rheumatism, head lice, alcohol poisoning, warts, sciatica, etc. Examples of product use:
- High temperature rubdown. It is better to use natural rice, apple or wine vinegar, but you can also use regular table vinegar (6 or 9%). For 0.5 liters of warm water, add 1 tbsp. l. vinegar, mix the composition, and then use for rubbing.
- Remedy for atherosclerosis. From 4 heads of garlic and 5 lemons, you need to squeeze the juice, mix the components with 0.5 liters of honey and 50 ml of vinegar (apple cider). You need to take the composition in 1 tbsp. l., mixed with ½ tbsp. water, three times a day. The course of therapy is 3 months.

In cosmetology
The product has shown its effectiveness in the fight against excess weight and sagging skin. A course of body wraps with vinegar allows you to almost completely eliminate cellulite. In addition, it is known about the use of liquid for the treatment of acne, acne and dandruff: this result is achieved due to the bactericidal properties of vinegar. Examples of product applications:
- Acetic peeling. Gauze folded in several layers is dipped in slightly warmed wine vinegar (you first need to make slots for the lips and eyes). The compress is placed on the face for 10 minutes. After removing the material, you need to walk for another hour without washing. After that, you need to take a napkin or a sponge of medium hardness, wipe your face with it, and then wash your face with cool water.
- A remedy for calluses. Mix 1 liter of warm water with 0.5 tbsp. apple cider vinegar and 1 tbsp. l. baking soda... Legs soar for at least 15 minutes, after which keratinized tissues are easily removed with the help of pumice.
Video
Candidate of Biological Sciences N. KUSTOVA
Many dishes in the cuisines of different nations of the world cannot be prepared without vinegar. You cannot do without it in blanks, and as a simple seasoning, vinegar is served with many dishes. The making of vinegar, like winemaking, is one of the oldest technological processes mastered by man. But if in the production of wine over the past few millennia there have been no fundamental changes (the use of modern equipment does not count), then in the production of vinegar in the 70s of the twentieth century, a real revolution took place.
Rice. 1. Schutzenbach's apparatus: 1 - wooden conical container; 2 - a layer of beech shavings.
![]()
Rice. 2. Frings apparatus: 1 - body; 2 - false perforated bottom; 3 - a layer of beech shavings; 4 - circulation pump; 5 - coil of the thermostatting system; 6 - switchgear.
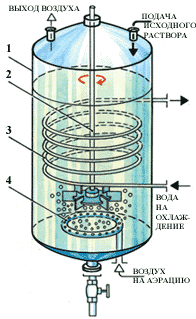
Rice. 3. Diagram of a fermenter for the production of vinegar: 1 - stainless steel body; 2 - mixing device; 3 - aerator (it is usually called a bubbler); 4 - coil of the thermostatting system.

Rice. 4. Scheme of the installation for obtaining vinegar in a continuous mode. The overflow of liquid from the apparatus to the apparatus occurs due to the pressure difference in the "air cushion" arising from the different depths of the overflow pipes h: h2> h3> h4> h5.
To begin with, the main ingredient in edible vinegar is acetic acid. It can be obtained in two ways: chemical - from the products of dry distillation of wood and microbiological - as a result of acetic acid fermentation of alcohol-containing liquids, such as grape wine, cider, beer wort, fermented honey and juices of various fruits or an aqueous solution of ethyl alcohol (C 2 H 5 HE). In such liquids, the oxidation of ethanol to acetic acid is carried out in most cases by acetic bacteria. Acetobacter aceti. As a result, the finished product contains not only acid, but also a small amount of esters, aldehydes and other organic compounds. It is thanks to these substances that food vinegar acquires its inherent special taste and pleasant aroma. Acetic acid diluted with water, obtained by chemical means, is devoid of such qualities. It is believed that in the food industry and in everyday life, it is better to use vinegar made by a biochemical method.
Vinegar production technology has an interesting and complex history. Back in the first millennium BC, winemakers noticed that if wine is left in an open vessel, after a while it turns sour and turns into vinegar. This observation was used for a long time, without going into the essence of what happens with the product.
One of the most "ancient" methods of vinegar production is called Orleans. At the beginning of the process, 10-12 liters of ready-made unfiltered vinegar are poured into wooden barrels of a special shape, located in a warmed room in several rows one above the other. This portion is a kind of leaven, because unfiltered vinegar contains a fairly large number of bacteria. About 10 liters of filtered wine are poured into the vinegar. After eight days, if the process goes well, add another 10 liters, and so on until the barrel is half full. After that, about 40 liters of the finished product is drained, and filtered wine is added to the remaining one, and the cycle is repeated. The whole cycle takes from a week to a month, but the product is of such high quality that this ineffective method is still used in the wine-growing regions of France.
Along with the Orleans method, there was a method described by the German scientist Boerhave in 1732. This technology is now known as the Schutzenbach method. Its essence is that an alcohol-containing liquid (in the description of Burgav, a solution of bread alcohol is mentioned) was passed from top to bottom through a volume filled with large beech shavings carefully soaked in vinegar. This technology turned out to be significantly more productive than the Orleans method, and it is still used all over the world.
And yet, until the work of Pasteur in the middle of the 18th century, it was not clear how wine turns into vinegar. Pasteur in his large article "Investigation of the properties of vinegar" ("Etude sur le vinaegre") showed that a sterile solution of alcohol in water in the open air practically does not oxidize, and the formation of acetic acid occurs due to the work of acetic bacteria. And in order for alcohol to oxidize effectively, it is necessary to create optimal conditions for their development in the liquid. It turned out that these microorganisms feel best of all at a temperature of about 30 ° C and at an alcohol concentration not exceeding 12-14%. Further (already modern) studies have shown that the maximum growth rate A.aceti is achieved at a lower alcohol concentration. A characteristic feature of these bacteria is their high oxygen demand. For a long time it was believed that due to the relatively low solubility of oxygen in water (and in a solution of ethyl alcohol too), bacteria can develop only on the surface of a liquid or in its thin film. This did not contradict the industrial experience available at that time. In the Orleans method, bacteria develop mainly in the upper layer of the liquid in the form of a slimy film, while in the Schuzenbach method, the liquid flows down in a thin layer over the surface of the shavings (Fig. 1). The productivity of the apparatus, in one way or in another, is usually from 2 to 8 kg of 100% acetic acid from 1 m 3 of the volume of the apparatus per day.
The main apparatus in which acetic acid is obtained by the Schuzenbach method is a conical wooden vat. At a distance of 200-300 mm from the main bottom, a horizontal perforated partition is installed in it. The upper part of the apparatus is 2/3 filled with shavings, which are irrigated with a nutrient medium for bacteria, containing a certain amount of acetic acid (most often this is a 6% solution), ethyl alcohol (3-4%) and a small amount of ammonium and phosphate salts. As the solution proceeds, bacteria that have become entrenched, or, as it is now customary to say, immobilized on the shavings, oxidize alcohol to acetic acid. In the lower part of the device, finished products- 9% vinegar. In the process of oxidation, heat is released, which raises the temperature inside the apparatus to 30-35 o C. As a result of the temperature difference, natural and rather intense convection is created. Air enters the pipes under the false bottom, passes through the apparatus and exits at the top of it. This is how the aeration necessary for the bacteria to work is carried out by itself.
A few words should be said about shavings. This is not just waste from wood processing. Only beech shavings rolled into a roll with a diameter of 2 to 5 cm and a height of 3 to 6 cm are suitable for loading into the devices. Serious requirements are imposed on wood. It must be completely free from any kind of rot. In a word, shavings for vinegar production are not at all cheap.
The Schutzenbach apparatus is loaded with 1-1.5 m 3 of shavings. Dozens of such devices operate at one enterprise. The productivity of the equipment when operating according to this method is low and amounts to no more than 1.5 kg of acetic acid per 1 m 3 of shavings per day (in terms of 100% acetic acid). In this case, the yield of vinegar (from the theoretically possible when using the initial amount of ethyl alcohol) does not exceed 75%. The process is carried out continuously, for decades, without changing bacteria and chips. The high acidity of the solution poured into the apparatus is necessary so that other bacteria cannot "populate" the apparatus and thus spoil the product. This makes it possible to carry out the production of vinegar without observing sterility. The only companion of vinegar bacteria in this process are small nematodes - eels. They feed on bacteria and also easily tolerate high concentrations of acetic acid. Vinegar is purified from them by filtration after pasteurization, as a result of which they die and precipitate.
At present, the overwhelming majority of enterprises produce vinegar using the Frings circulation method. This technology has a lot in common with the Schutzenbach method. Here, devices filled with shavings are also used, acetic acid bacteria are also immobilized on the shavings, and the mass of shavings is also irrigated with a nutrient solution containing alcohol, acetic acid and mineral salts. However, there are also significant differences between these methods. First of all, it concerns the size of the devices. In some enterprises, the volume of their working chamber filled with shavings reaches 60 m 3. A 10% alcohol solution is fed into such an apparatus (Fig. 2) through a special distribution system at a rate several times higher than according to the Schuzenbach method. Using a pump, the solution is repeatedly circulated through the apparatus until all the alcohol is oxidized and a 9% acid solution is formed. About 10% of the original pure alcohol is lost in this process. The cycle lasts 5-6 days, after which it is repeated.
In devices of a large volume, the heat release turns out to be so significant that special heat exchangers have to be built into them. Most often, coils are located in the working chamber through which cooling water circulates, but sometimes it is necessary to arrange additional, so-called external heat exchangers, which are installed outside the apparatus in the circulation circuit.
When vinegar is obtained by the circulation method, the specific productivity reaches 6-8 kg of acid per day per 1 m 3 of the working volume of the apparatus.
But this method also had significant drawbacks, the main of which was, perhaps, the size of the apparatus. In the early sixties of the twentieth century, a technology appeared, in which acetic acid bacteria began to be cultivated in special apparatus - fermenters in liquid, - the so-called method of periodic submerged cultivation.
Fermenters for submerged cultivation of acetic bacteria are containers made of stainless steel, inside of which there are mixing devices and aerators of various designs (Fig. 3).
The process of obtaining vinegar with the periodic deep method is as follows. From the previous cycle, liquid remains in the apparatus (approximately 1/3 of the working volume of the apparatus), which serves as the seed for the next cycle. A nutrient mixture containing acetic acid and ethanol is poured into the apparatus up to the working volume. The stirring device intensively mixes the liquid, and air is continuously supplied through the aerator. At the beginning of the cycle, the living conditions for bacteria change dramatically, and as a result, their noticeable growth is not observed for some time, this stage in the development of microorganisms is called the lag phase. At the end of the lag phase, the alcohol concentration begins to decrease, while the acid concentration, on the contrary, increases. For some time, a solution of alcohol has to be added to the apparatus in portions. After the concentration of vinegar reaches 9-10%, about 2/3 of the volume of the liquid is taken as a finished product, and the cycle is repeated.
The productivity of downhole devices is several times higher, and they themselves are several times less than devices filled with shavings, they have significantly less ethanol loss. In addition, there is no need to use wood shavings. It is also important that the production culture increases with the deep method.
In the early 70s of the last century, a group of employees of the Department of Machines and Apparatus for Microbiological Production at the Moscow Institute of Chemical Engineering (now it is the Moscow State University of Engineering Ecology), headed by Professor Peter Ivanovich Nikolaev, had an idea to combine microbiological methods with techniques on an industrial scale setting up and conducting processes well developed in chemical technology. For this, a whole complex of serious research had to be carried out. Here's a paradox: the process has been known for at least two and a half millennia, but until the middle of the twentieth century it remained mostly empirical. Up to this point, improvements in technology concerned primarily the device design, and microbiological aspects were developed very poorly.
In the 60s, works began to appear on the physiology and biochemistry of acetic bacteria. They were aimed at studying the influence of oxygen concentration and the composition of the nutrient medium, including both the mineral background and the effect of ethanol and acetic acid itself. At the same time, studies of the taxonomy, morphology and physiology of these bacteria were carried out at the Department of Microbiology of Leningrad University under the guidance of Professor M. S. Loitsyanskaya. Were isolated strains of bacteria growing in a very simple in composition medium with high oxidative activity, which turned out to be extremely useful for industrial production vinegar.
Optimum temperature for growth Acetobacter aceti - 25-30 o C. As a nitrogen source, acetic acid bacteria use mineral salts, preferably ammonium. Acetobacters themselves synthesize all the necessary vitamins and therefore grow in nutrient media without their addition.
The best carbon compound for bacteria of the genus Acetobacter is acetic acid. They also grow well in environments containing ethyl alcohol or lactic acid, converting them into acetic acid.
Studies by Yu. L. Ignatov have shown that the acetic acid accumulated in the process reduces the oxidative activity of bacteria and reduces the specific rate of cell growth. This fact allowed P.I. Nikolaev and his co-workers to organize the process of obtaining acetic acid in a battery from several devices by a deep method in a continuous mode. The result is the original technology system, in which the process of obtaining 9% acetic acid is carried out in four to five series-connected fermenters (Fig. 4). In such a battery, in the first two, in the course of the liquid, devices with a relatively low concentration of acetic acid, bacteria multiply at a high rate with high oxidative activity, which ensures high productivity of the process. On the other hand, in the last apparatus along the flow of liquid, operating at high concentrations of acetic acid, productivity decreases, in them there is mainly additional oxidation of the alcohol remaining in the solution. The overall performance of all battery apparatus is significantly higher than one that produces 9% vinegar. Yu.L. Ignatov showed that the productivity of a unit of the working volume of a battery-operated apparatus can reach 49.4 kg of acetic acid from 1 m 3 per day.
The developed method was implemented surprisingly quickly in several factories. Now this technology is used by the Experimental Food Processing Plant in Balashikha, vinegar shops in the cities of Gorlovka and Dneprodzerzhinsk in Ukraine, and a plant in Slovakia.
N. KUSTOVA, Candidate of Biological Sciences, Associate Professor of the Moscow State University of Environmental Engineering. Details for the curiousBrief information on the chemistry of ethanol oxidation to acetic acid Acetobacter aceti
The final reaction of the oxidation of ethyl alcohol to acetic acid is as follows:
Acetobacter aceti
C 2 H 5 OH CH 3 COOH + H 2 O + Q
According to modern concepts, the oxidation of ethyl alcohol by acetic acid bacteria of the type Acetobacter aceti - two-phase process. Ethanol is oxidized by alcohol and aldehyde dehydrogenases to form acetic acid and two NADH 2 molecules. (This enzyme is responsible for the transport of hydrogen in the respiratory chain.)
Alcohol dehydrogenase Acetobacter aceti contains the recently discovered prosthetic group methoxanthine, or pyrroloquinoline quinone. This enzyme is located on the outside of the plasma membrane and catalyzes the oxidation of ethanol to acetic acid. Methoxanthin is partially absorbed into the culture medium and into food vinegar, giving it a slightly yellowish color.
First of all, acetic acid, at a concentration close to 100 percent, is called glacial, while a 70-80 percent aqueous solution of this acid is called acetic essence. It is impossible not to mention the 3-15% solution known as table vinegar. It is noteworthy that aqueous solutions of acetic acid are widely used not only in the food industry (E260), but also in household cooking, in particular in canning.
Meanwhile, vinegar essence is the trade name of an 80% aqueous solution of food-grade acetic acid, obtained by the industry by fermentation of alcoholic liquids with acetic acid. Vinegar essence is usually used in the preparation of table vinegar, pickles and canned foods.
Often, vinegar essence is needed for blanks, and you only have 9% table vinegar in stock, or vice versa. Many housewives do not know how to get out of the situation, but in fact these fluids are interchangeable. The main thing is to know the correct proportions.
For example, in order to obtain 70% of the vinegar essence with ordinary table vinegar, it is necessary to observe the following proportions:
3% vinegar = 1 tbsp. l. essences for 20 tbsp. l. water,
6% vinegar = 1 tbsp. l. essences for 11 tbsp. l. water,
9% vinegar = 1 tbsp. l. essences for 7 tbsp. l. water.
Conversely, 70% vinegar essence can be easily obtained from table vinegar, however, this requires reducing the amount of water specified in the recipe by as much as the vinegar is supposed to be added. The calculation formula is pretty simple:
1 tbsp. l. essences = 8 tbsp. l. 9% vinegar for 7 tbsp. l. water,
1 tbsp. l. essences = 12 tbsp. l. 6% vinegar for 11 tbsp. l water
1 tbsp. l. essences = 21 tbsp. l. 3% vinegar for 20 tbsp. l. water.
Vinegar essence is considered a fairly toxic substance, poisoning with which is one of the most common household intoxications. So, a lethal dose in the absence of immediate assistance is from 30 to 50 milliliters of vinegar essence 80%.
In the case of using pure vinegar essence, severe burns of the mucous membranes of the oral cavity and pharynx occur, and the esophagus and stomach also suffer. The consequences of the absorption of vinegar essence include diseases such as hemoglobinuria, hemolysis, acidosis, bleeding disorders, accompanied by severe bleeding in the stomach and intestines. That is why, in order to avoid such problems, vinegar essence should be used in a diluted form and in limited quantities.
Caloric content of Acetic essence is 11.3 kcal. Energy value of the product Acetic essence (The ratio of proteins, fats, carbohydrates):
Proteins: 0 g (~ 0 kcal)
Fats: 0 g (~ 0 kcal)
Carbohydrates: 3 g. (~ 12 kcal)
Energy ratio (b | f | y): 0% | 0% | 106%
Vinegar Essence Recipes

pickled cucumbers with red currants Marina Pugovkina | 14.10.2014 | 1647
Marina Pugovkina 10/14/2014 1647

Let's see what is interesting about this product, as well as what is the difference between natural vinegar and "synthetics".
We are accustomed to the fact that vinegar is a liquid with a pungent smell, which is used for conservation. In fact, this product is not that simple.
Natural and synthetic vinegar - what's the difference?
Vinegar can be natural or synthetic. The first has a noble origin, to match the guilt. It contains many vitamins and useful microelements, helps to cleanse the body, so it must be included in the diet.
Natural vinegar includes wine, balsamic, fruit and berry, apple, alcohol. These seasonings with a small percentage of acid and alcohol give dishes a unique sourness and new shades of taste and aroma.
Vinegar obtained by diluting concentrated synthetic acetic acid has no aromatic properties, but it has a specific smell of this acid. It contains unhealthy aldehydes and heavy metal salts, so synthetic vinegar is best used for various household purposes, and not in cooking.
Read the label carefully to tell the difference between synthetic and natural vinegar. The inscriptions: "essence", "acetic acid" (70-80%) or "table vinegar" mean that this is a synthetic product. On the label of real vinegar it is written "natural vinegar" or "biochemical", "alcoholic".
Natural vinegar is divided into types depending on the raw materials from which it is made. Most often, the following types are found on the shelves.
Natural apple cider vinegar is made from fermented apple cider juice. The color can range from light gold to rich amber.
To buy good apple cider vinegar, you need to find out if it has been pasteurized or not. If during production Apple juice not exposed heat treatment Most of the vitamins and microelements contained in apples are preserved.
Another way to test the quality of this vinegar is to shake the bottle. If the foam disappears immediately, within three seconds, natural vinegar. In an artificial one, the foam lasts longer, up to 10 seconds.
Be careful! The inscription on the label "Apple" is not a guarantee that the vinegar is real. If the composition contains apple flavoring, then it is ordinary table vinegar, which "pretends" to be a natural product.
For a long time, vinegar of this type was considered a by-product of winemaking and was not used in cooking. Essentially, wine vinegar is fermented grape wine.
Depending on the grape variety, it can be white or red. It is an ideal base for sauces, marinades, dressings. When buying wine vinegar, make sure that there is no sediment at the bottom of the bottle, and that there is no foam at the top of the bottle. Their presence indicates the spoilage of the product.
Balsamic vinegar is a type of grape vinegar. It is made from white grape varieties with a high sugar content.
The cooking technology is quite complicated, it can take 12, 25 and even 100 years. Only 3 liters of precious "black gold" is obtained from 100 kg of grapes, therefore real balsamic vinegar is an exclusive and very expensive product. It is dark brown in color and viscous consistency, and on the packaging has the mark of European quality certification - DOP.
How to choose the right vinegar?
- Opt for vinegar in a glass bottle. Plastic containers are not intended for long-term storage.
- Good vinegar (with the exception of wine) should have a slight sediment at the bottom of the bottle, which indicates the naturalness of the product.
- If the composition contains dyes, preservatives, caramelized sugar, concentrated juice - this is low-quality vinegar.
- For blanks, it is better to choose natural alcoholic 9% vinegar. It is made from grain ethyl alcohol. It is transparent and colorless, like synthetic (table). But with it, the workpieces will turn out to be more delicate in taste, and there will be no chemical aroma.
How to store vinegar?
Store the vinegar in a tightly closed container in a cool, dark place. A food cabinet located away from heat sources such as a stove, hob or radiators is best suited for this. A vinegar refrigerator is not suitable.
Do not heat vinegar. Add it to hot dishes at the very last moment.









